The South African Health Products Regulatory Agency has collaborated with Bayer Ltd to issue a recall for a specific batch of Yaz Plus contraceptive pills, prompting health officials to advise women using the potentially ineffective pills to seek medical consultation.
Contraceptive Pill Recall: Bayer's Yaz Plus Faces Packaging Mix-Up in South Africa
Contraceptive Pill Recall: Bayer's Yaz Plus Faces Packaging Mix-Up in South Africa
A recall has been initiated for a limited batch of Yaz Plus contraceptive pills due to an incorrect packaging issue that could compromise contraceptive effectiveness.
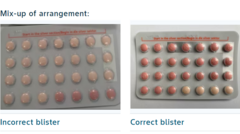
Regulators in South Africa are alerting users about a recall of a batch of the widely used Yaz Plus contraceptive pill, due to a packaging discrepancy that raises concerns about its effectiveness. Bayer Ltd, the manufacturer, reported that specific blister packs were improperly organized, resulting in some containing 24 inactive pills rather than the expected 24 active hormone pills. Women are urged to stop using these pills immediately and consult healthcare professionals for guidance.
The issue revolves around a limited batch marked WEW96J, which has an expiry date of March 2026. In this problematic batch, some packs mistakenly featured all hormone-free pills, which could inadvertently put women at risk of unintended pregnancy due to reliance on what they believed were effective contraceptive measures.
Bayer Ltd, in partnership with the South Africa Health Products Regulatory Agency, has taken necessary actions to recall these items. They reassured the public that the "root cause" of the packaging error has been identified and addressed.
Typically, Yaz Plus contraceptives come with a regimen of 24 pink hormone-containing active pills, followed by four inert light orange pills. However, the affected batch deviated from this standard, carrying only inactive pills for the majority of the pack.
As a preventive step, Bayer advises that no pills from this recalled batch should be taken until a healthcare practitioner is consulted. Users of the affected pill packs are encouraged to return them to pharmacies for a refund or replacement. Healthcare providers, pharmacies, and wholesalers that might still hold these faulty packs are also instructed to return them.
Bayer Ltd has initiated a helpline specifically for inquiries related to this recall, aiming to assist anyone with concerns surrounding the affected contraceptive pills. The company has emphasized that only the specified batch is affected and that all other batches remain safe for use.


















