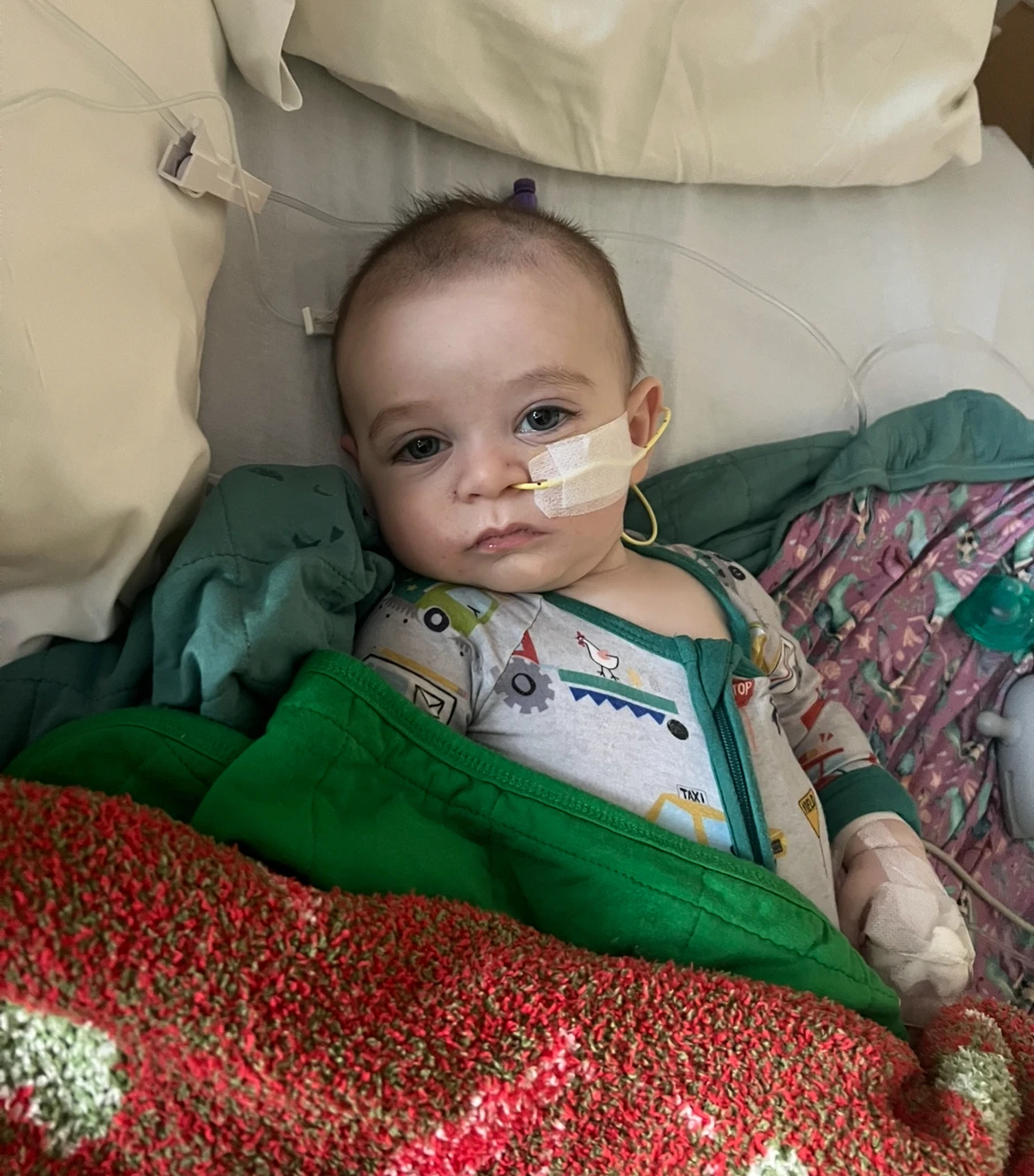
The parents of two infants, both diagnosed with infantile botulism, have initiated legal action against the manufacturers of ByHeart baby formula, at the center of a significant nationwide recall. Stephen and Yurany Dexter from Flagstaff, Arizona, reported that their daughter, Rose, required airlifted medical attention to treat the life-threatening condition.
Similarly, Michael and Hanna Everett from Richmond, Kentucky, rushed their daughter, Piper, to a hospital as her health deteriorated. The families are seeking compensation for extensive medical bills, emotional trauma, and damages, claiming that ByHeart's formula was defective and the company acted negligently.
Both families chose the organic formula, seeking a natural option for their children, but were devastated to see their infants suffer. I wouldn’t have guessed that a product for such a vulnerable child could cause something so severe, remarked Stephen Dexter.
Nationwide Health Concerns
This outbreak has reportedly affected at least 15 children across the U.S., beginning in August 2023. The only treatment for infantile botulism, a rare but potentially fatal illness, is a specialized IV medication known as BabyBIG, derived from the blood of immunized donors. Authorities have confirmed traces of botulism-causing bacteria in samples from the recalled ByHeart formula.
As investigations continue into additional suspected cases, ByHeart has ceased distribution of its products nationwide, though the company has stated it will respond to the lawsuits in due time. With over 200,000 cans sold every month, this incident has raised significant health and safety concerns among parents using similar products.
We strive to provide safe, organic nutrition for our children, shared Hanna Everett, reflecting parents' concerns about the trustworthiness of baby formula brands. As more families come to terms with the ramifications of this outbreak, experts believe these initial lawsuits may herald a broader wave of legal action against ByHeart.