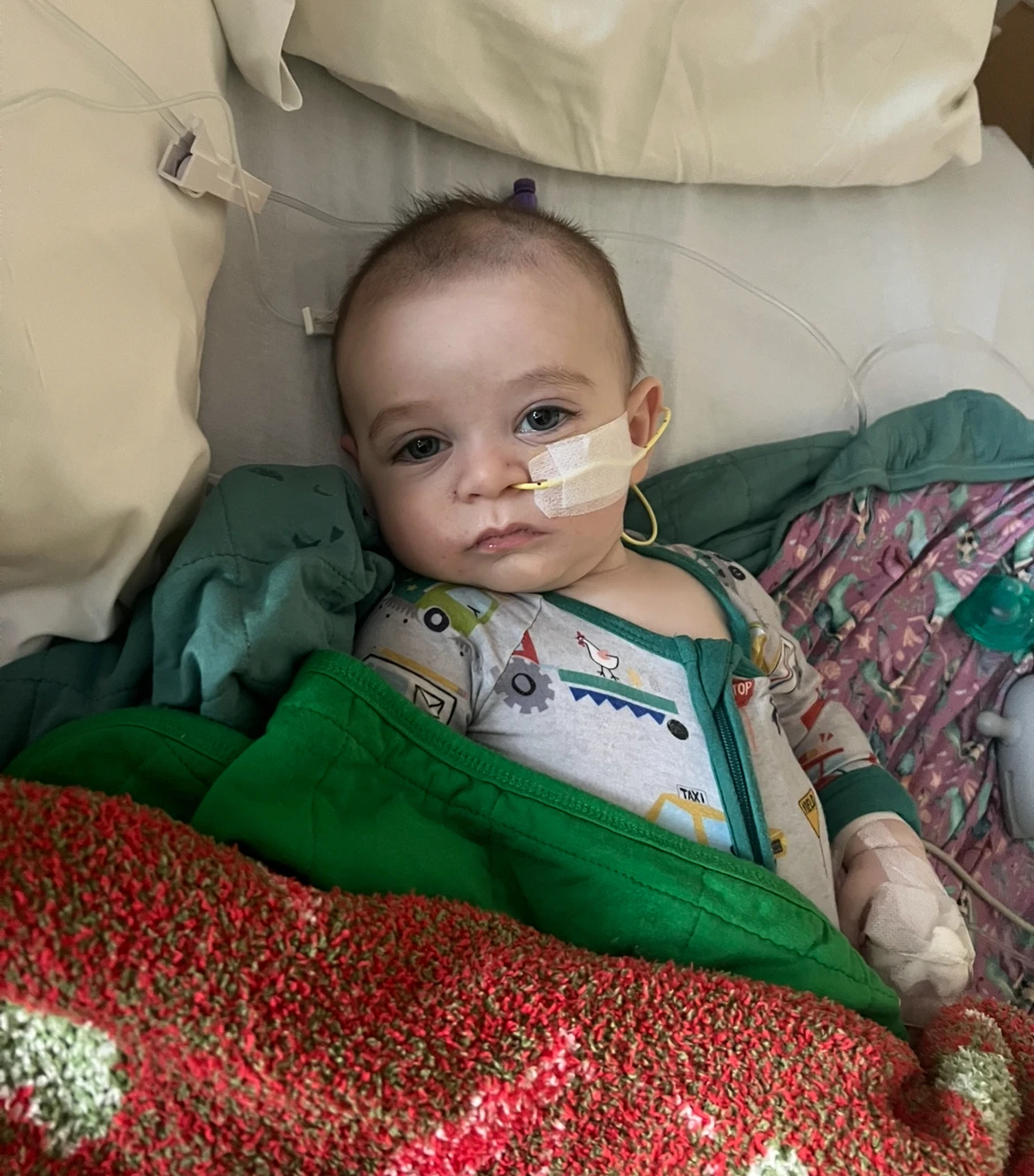
Tests conducted on ByHeart infant formula have linked its products to a botulism outbreak that has resulted in illnesses among dozens of babies. Lab tests of multiple formula samples demonstrated the presence of bacteria that could lead to this rare yet potentially lethal illness, prompting health officials to declare all ByHeart products at risk of contamination.
At least 31 infants across 15 states have been affected by this outbreak, which began in August. Notably, some cases have been traced back to earlier months, raising concerns about potential underreporting of the illness.
Clostridium botulinum type A, the harmful bacteria found in the formula, can be unequally distributed within powdered products, making it difficult to predict which containers may carry the pathogen. Medical experts emphasize that all infants under the age of one face increased risk if exposed.
In light of these findings, ByHeart issued a nationwide recall on November 11. However, investigations reveal that some of the recalled items remain available on retail shelves. Parents are urged to cease use of these products immediately and monitor their children for any signs of illness, which may take up to a month to manifest.
Infant botulism arises when spores are ingested, leading to toxin production in the intestines. Symptoms include constipation, difficulty feeding, and unusual facial expressions, with serious cases requiring emergency medical intervention.
To report any related illness, affected individuals can contact an FDA consumer complaint coordinator or use the online MedWatch form for official documentation. Families purchasing ByHeart products directly from the company's website since August can request full refunds, responding to recent consumer concerns.