Article text:
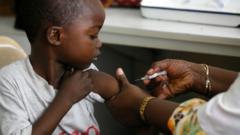
The first-ever malaria treatment specifically formulated for infants has received regulatory approval, marking a significant advancement in global health. This much-anticipated medication is set to be rolled out across various African nations within weeks, providing hope for the youngest and most vulnerable populations.
Historically, while malaria treatments existed for children, there was a lack of options designed explicitly for babies. Pre-existing medications meant for older children posed significant risks of overdose, given that their dosages may not align with the needs of infants whose development is still in progress. Unfortunately, in 2023, malaria was responsible for approximately 597,000 deaths worldwide, with a staggering number of these fatalities—around 75%—affecting children under five in Africa.
The newly approved medication, named Coartem Baby (or Riamet Baby in certain regions), was jointly developed by Novartis and the Medicines for Malaria Venture (MMV). This collaborative effort has been endorsed by several international organizations and governments, further emphasizing the urgency of this health crisis. By introducing this medication on a largely not-for-profit basis, Novartis aims to ensure that one of the most susceptible demographics gains access to essential life-saving treatment.
Vas Narasimhan, CEO of Novartis, has expressed optimism, stating that this achievement is the culmination of over thirty years of dedication to combating malaria. He emphasized the need for targeted and effective treatments to cater to the most vulnerable sections of the population.
The endorsement from MMV is equally significant, as CEO Martin Fitchet underscored the importance of this drug in addressing the high mortality rate of malaria, particularly among children. He believes that with the proper focus and resources, malaria can be effectively eliminated.
Experts are hopeful that this innovative treatment will lead to a dramatic decrease in malaria-related fatalities, especially in sub-Saharan Africa, where the death toll is critically high. Health professionals view the introduction of Coartem Baby as a major breakthrough in the fight against malaria, which poses an especially lethal threat to infants, particularly those with underlying health conditions such as sickle cell disease.
The commitment from Novartis to provide this drug at minimal cost could play a crucial role in leveling disparities in healthcare access, ultimately saving lives and reducing the burden of malaria in affected regions.
The first-ever malaria treatment specifically formulated for infants has received regulatory approval, marking a significant advancement in global health. This much-anticipated medication is set to be rolled out across various African nations within weeks, providing hope for the youngest and most vulnerable populations.
Historically, while malaria treatments existed for children, there was a lack of options designed explicitly for babies. Pre-existing medications meant for older children posed significant risks of overdose, given that their dosages may not align with the needs of infants whose development is still in progress. Unfortunately, in 2023, malaria was responsible for approximately 597,000 deaths worldwide, with a staggering number of these fatalities—around 75%—affecting children under five in Africa.
The newly approved medication, named Coartem Baby (or Riamet Baby in certain regions), was jointly developed by Novartis and the Medicines for Malaria Venture (MMV). This collaborative effort has been endorsed by several international organizations and governments, further emphasizing the urgency of this health crisis. By introducing this medication on a largely not-for-profit basis, Novartis aims to ensure that one of the most susceptible demographics gains access to essential life-saving treatment.
Vas Narasimhan, CEO of Novartis, has expressed optimism, stating that this achievement is the culmination of over thirty years of dedication to combating malaria. He emphasized the need for targeted and effective treatments to cater to the most vulnerable sections of the population.
The endorsement from MMV is equally significant, as CEO Martin Fitchet underscored the importance of this drug in addressing the high mortality rate of malaria, particularly among children. He believes that with the proper focus and resources, malaria can be effectively eliminated.
Experts are hopeful that this innovative treatment will lead to a dramatic decrease in malaria-related fatalities, especially in sub-Saharan Africa, where the death toll is critically high. Health professionals view the introduction of Coartem Baby as a major breakthrough in the fight against malaria, which poses an especially lethal threat to infants, particularly those with underlying health conditions such as sickle cell disease.
The commitment from Novartis to provide this drug at minimal cost could play a crucial role in leveling disparities in healthcare access, ultimately saving lives and reducing the burden of malaria in affected regions.